Your Intelligent Platform to Fast-Track Regulatory Approval Processes of Drugs and Medical Devices.
Perfect your new drug approval applications with Regucy Advisory Group
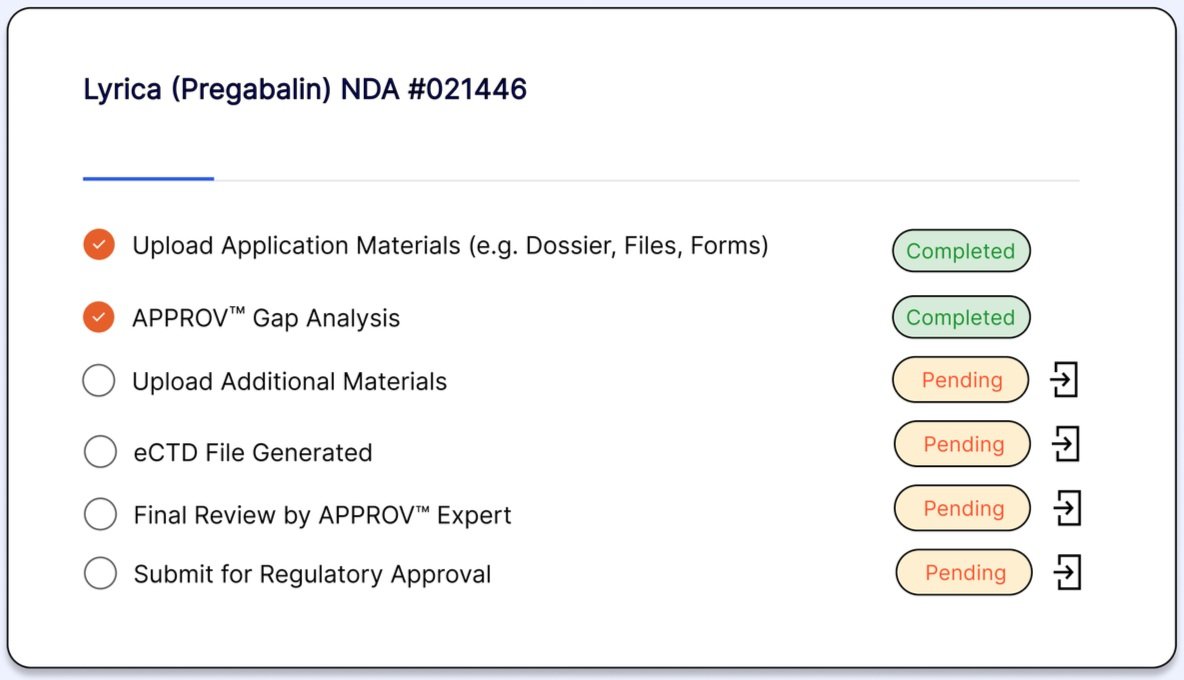
Our global team is dedicated to identifying weaknesses in your draft submission.
Proactively identify gaps and missing requirements
Regucy presents you with a seamless experience in your journey to perfect your new drug applications.
Navigate the complexities of drug approval with ease, as Regucy's Regional Regulatory Advisors offer tailored guidance and insights specific to your need.
Regucy Advisors
-
At Regucy Advisory Group, we simplify the complexities of Clinical Trials through our expertise with Health Canada, FDA, and EMA.
Our Services Include:
Clinical Trial Applications: Accurate submissions for Health Canada CTAs and US INDs.
Product Evaluation: Gap analyses and strategic regulatory guidance.
Market Authorization Holder: Management of your market authorization and renewals.
Label Review: Compliance with labeling standards.
Facility Registration: Streamlined FDA registration processes.
Multilingual Translation: Ensuring your regulatory documents meet global standards.
Start your journey towards successful regulatory submssion today. Learn more
-
Elevate your regulatory submissions with Regucy Advisory Group’s cutting-edge eCTD publishing and AI integration.
Key Features:
Tailored Submissions: Customized for your product needs.
Expert Authoring: Comprehensive CTD Modules and risk management plans.
Efficient Assembly: Streamlined for global compliance.
AI Precision: Enhanced formatting and metadata management.
Dedicated Support: Our AI experts ensure quality and satisfaction throughout the process.
Begin your seamless eCTD journey today. Learn more
-
Regucy Advisory Group offers tailored GxP compliance services for the pharmaceutical and biotechnology sectors, including:
Good Manufacturing Practices (GMP): Comprehensive audits, mock inspections, SOP development, and quality system implementation.
Good Clinical Practices (GCP): In-depth audits, training for clinical staff, and regulatory document reviews.
Partner with Regucy to uphold the highest standards of quality and safety.
Discover how we can enhance your compliance. Learn more
-
Regucy specializes in global cosmetics registration, ensuring your products meet the highest regulatory standards through:
Product Compliance: Ingredient reviews, labeling checks, and lab testing for safety and adherence to regional regulations.
Regulatory Submission: Expert documentation preparation and tailored strategies for seamless communication with authorities.
Post-Registration Monitoring: Ongoing compliance monitoring to maintain regulatory adherence.
Partner with Regucy for a smooth and efficient registration process.
Ensure your cosmetic products meet international standards. Learn more
-
ISO certification is a hallmark of professional integrity, demonstrating compliance with internationally recognized standards. It enhances your organization’s marketability and opens doors to new opportunities by assuring stakeholders of consistent quality and reliability.
At Regucy Advisory Group, our expert ISO consultants guide you through the complex certification process, offering tailored solutions and comprehensive training. We provide a diverse range of ISO certification services, including ISO 9001, ISO 14001, ISO 27001, and more, tailored to various industries.
Our hassle-free ISO auditing services ensure compliance while identifying areas for improvement. We also offer support throughout the ISO accreditation process and provide an efficient virtual application process for your convenience.
Elevate your organization’s standards and marketability. Learn more
-
Regucy Advisory Group specializes in ensuring compliance with GMP regulatory standards, including China NMPA, FDA, EMA, and country-specific regulation. Our services include gap analysis, third-party audits, mock inspections, CAPA, and comprehensive support during inspections. We help manufacturers achieve and maintain the highest standards of product quality and regulatory compliance.
Discover how we can support your GMP third party audit in China. Learn more